HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention. Beckman Coulter has been providing cost effective solutions to monitor CD4 status in resource limited countries for several decades. The mounting HIV problem in these regions of the world has led to innovative solutions with a mission to deliver patient care.
|
‘Since it is so simple and intuitive, training my techs was a breeze. You could train them in less than an hour. So we were able to cross-train most of the techs in the area in very short time. That makes everything efficient in the laboratory.’ Alexandra Amador, MT (ASCP), CHT (ABHI), Manager Histocompatibility Lab, University of Miami. |
Sample preparation and data management usually are the major bottlenecks in routine flow cytometry. Even with a semi-automated process, you and your team may spend hundreds of hours per year for tasks that don't add to your laboratory's productivity.
Work with a system that combines sample preparation, reagent management, barcode scanning and analysis in one compact platform.
- Provide a 24/7 flow cytometry service as the AQUIOS CL can be run by less experienced operators.
- Increase productivity with high-throughput performance that eliminates many of the least efficient features of existing systems.
- Minimize the potential for user error inherent in existing systems that require numerous manual steps to set up and run.
- Discover the AQUIOS Tetra System for Lymphocyte Subset Analysis, AQUIOS PLG or CD4 testing or automate your own user-defined assay with AQUIOS Designer Software.
- Train in as little as one day for skilled flow cytometrists with computer based training videos.
- AQUIOS CL with Tetra-1 has been accepted for the World Health Organization list of prequalified in vitro diagnostic products.
AQUIOS CL Workflow: Eliminate Steps With Every Sample
Modular flow cytometry systems might be effective for higher complexity applications, but aren‘t the best option for efficiently managing a high volume of routine applications. With AQUIOS CL, you can automate your most routine, repetitive tasks.
Figure 1. Total elapsed time from instrument startup to shutdown was analyzed for 10, 25, and 50 lymphocyte subset samples on AQUIOS CL vs. the alternative method (FC500 with TQ-Prep). AQUIOS CL reduced actual hands-on time by up to 92%1.
Figure 2. Time to first result (sum of cytometer startup & cleaning, quality control, and sample preparation time including time for analysis of the first sample) was analyzed for 10, 25, and 50 lymphocyte subset samples on AQUIOS CL vs. the alternative method (BD FACSCalibur plus SPA II system)2.
Talk to an expert about CD4 testing solutions
HIV Advanced Disease Management

HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention..
Fast Throughput Subset Analysis with AQUIOS CL
In the clinical management of immune deficiency diseases, accurately counting the absolute cell numbers of leukocyte subsets and measuring the percentage of individual subtypes in blood is critical.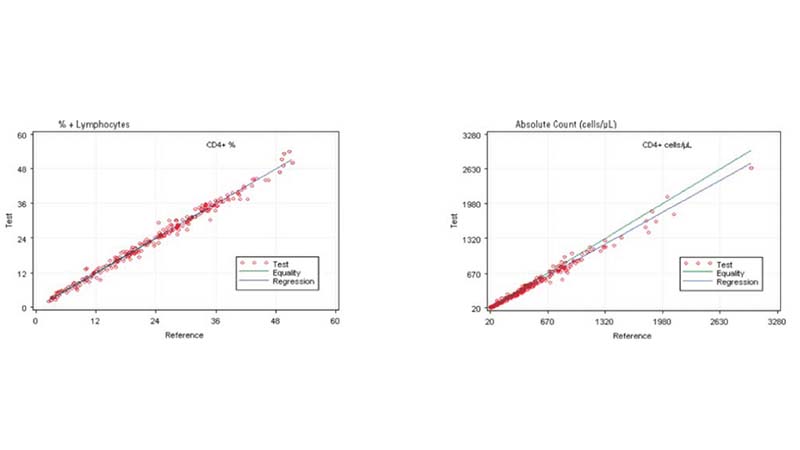
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL Tetra and Aged Whole Blood samples
Based on this study, the AQUIOS CL instrument with Tetra application provides accurate results for recovery of the T, B and NK lymphocyte subsets in clinical and normal specimens collected into the EDTA K3 tubes when stored at room temperature for up to 24 hours.Error Prevention on AQUIOS CL overview
Laboratory standards and regulations set forth by governmental bodies such as the Centers for Disease Control (CDC) help to define the procedures that laboratories follow
Error prevention during Startup and Worklist creation
The AQUIOS CL system employs several features that work to prevent errors during startup, cleaning, and worklist generation, including Autocleaning, Test Requests to and from the LIS and Creating Worklists.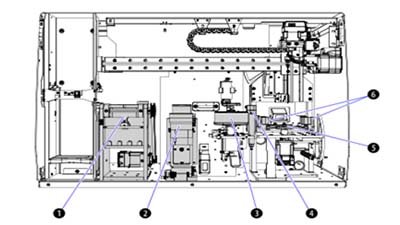
Error Prevention During Sample Prep
The AQUIOS CL system employs several features that work to prevent errors during sample prep.
Error Prevention During Quality Control
The AQUIOS CL system employs several features that work to prevent errors during quality control, including Automatically Pause when QC Fails, QC Checks, Daily QC (Controls), Real Time QC (Patient Sample).References:
- Reagents and sample material used: AQUIOS Tetra-1 with AQUIOS IMMUNO-TROL and AQUIOS IMMUNO-TROL Low cells (AQUIOS CL); CYTO-STAT tetraCHROME CD45/CD4/CD8/CD3 with IMMUNO-TROL and IMMUNO-TROL Low cells (FC500 with TQ-Prep). Data: The London Health Sciences Centre (Ontario, Canada).
- Reagents and sample material used: BD MultiTest CD3/CD8/CD45/CD4 with BD Multi-Check and BD Multi-Check Low Control cells (FACSCalibur plus SPA II); AQUIOS Tetra-1 with AQUIOS IMMUNO-TROL and AQUIOS IMMUNO-TROL Low cells (AQUIOS CL). Data: University of Texas Medical Branch (UTMB), Galveston, Texas.




